Details of the Drug
General Information of Drug (ID: DMIGFOR)
| Drug Name |
I3C
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Indole-3-carbinol; 700-06-1; 3-Indolemethanol; INDOLE-3-METHANOL; (1H-Indol-3-yl)methanol; 1H-indol-3-ylmethanol; 3-Hydroxymethylindole; 1H-Indole-3-methanol; 3-Indolylcarbinol; Indinol; 3-(Hydroxymethyl)indole; 3-Indole methanol; Indole 3 carbinol; (1H-Indol-3-yl)-methanol; MFCD00005632; UNII-C11E72455F; CHEBI:24814; C11E72455F; NSC-525801; NCGC00090701-06; indol-3-ylmethan-1-ol; I0496; Indole-3-carbinol, 97%; SMR000385784; CCRIS 3261; EINECS 211-836-2; 1H-Indol-3-Yl-Methanol; NSC 525801; BRN 0121323; AI3-60090; 3-Indolecarbinol; 3-Indolylmethanol; Prevention 4 (indole-3-carbinol); indole-3-carbinole; zlchem 356; PubChem7265; 3-hydroxymethyl indole; Spectrum2_001710; Spectrum3_001973; ACMC-209oc7; DSSTox_CID_11458; DSSTox_RID_78876; DSSTox_GSID_31458; BSPBio_003573; MLS001333161; MLS001333162; SCHEMBL195520; SPECTRUM1505320; SPBio_001700; CHEMBL155625; 1H-Indole-3-methanol (9CI); 3-Phenoxybenzylaminehydrochloride; DTXSID7031458; GTPL10047; KBio3_002949; ZLC0198; HMS1789O22; HMS2235E10; HMS3369B02; HMS3651I18; HMS3749E07; ZINC158743; ACN-S002804; ACT03591; BCP00087; HY-N0170; INDOLE-3-CARBINOL (I3C); Tox21_400055; 9344AF; ANW-35813; CCG-38786; HSCI1_000097; NSC525801; s2313; SBB004095; AKOS001075120; AC-7583; CS-7780; DB12881; GS-0916; LS20980; MCULE-6344603304; SB14958; SDCCGMLS-0065970.P001; SDCCGMLS-0065970.P002; VI30396; Indole-3-methanol (Indole-3-carbinol); SMP2_000172; NCGC00090701-01; NCGC00090701-02; NCGC00090701-03; NCGC00090701-04; NCGC00090701-05; NCGC00090701-07; AK-53373; CAS-700-06-1; SY015976; AB0008317; DB-011567; A9256; FT-0615875; ST50308202; SW219849-1; I-2100; M-3233; A836732; SR-01000838318; Q1770257; SR-01000838318-3; BRD-K01815685-001-02-3; BRD-K01815685-001-07-2; Z85923165
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
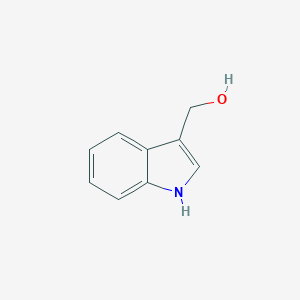 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 147.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
